Unlike bisphenol-A type epoxy resins, cycloaliphatic epoxy resins have epoxy groups in an electron-rich state, making them difficult to react with nucleophilic agents. Due to the presence of steric hindrance, nucleophilic agents have difficulty attacking the carbon atoms. Therefore, their reactivity is much slower than terminal epoxy groups, making cycloaliphatic epoxy resins hard to react with amine and imidazole curing agents.

However, when reacting with electrophilic agents, since the electrophilic agents attack the oxygen atoms, cycloaliphatic epoxy resins do not experience steric hindrance. Hence, cycloaliphatic epoxy resins easily react with polyols, anhydrides, and cationic curing agents.
| Amines | Phenols | Anhydrides | Cations | |
| Cure Difficulty | ★☆☆☆☆ | ★★☆☆☆ | ★★★☆☆ | ★★★★★ |
| Tg of Cured Resin | ★☆☆☆☆ | ★★☆☆☆ | ★★★★★ | ★★★★☆ |
| Color | ★☆☆☆☆ | ★★☆☆☆ | ★★★★★ | ★★★★☆ |
| Mechanical Properties | ★☆☆☆☆ | ★★☆☆☆ | ★★★★☆ | ★★★☆☆ |
Figure 1 compares the curing kinetics curves of cycloaliphatic epoxy resins S-06E, bisphenol-A type 128 epoxy resin, and alicyclic amine 1,3-BAC. From the figure, it is evident that due to the steric hindrance effect, nucleophilic agents have difficulty attacking the carbon atoms, making S-06E and 1,3-BAC harder to react. The initial reaction temperature and peak reaction temperature are both much higher than those of 128 + 1,3-BAC.
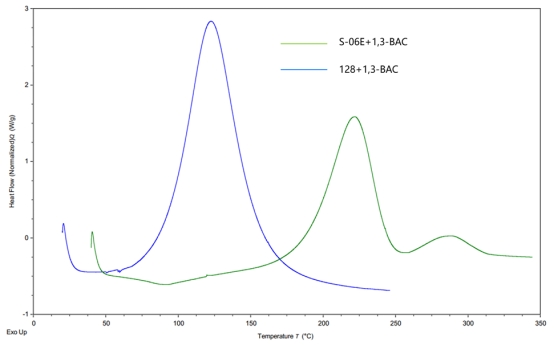
Figure 1
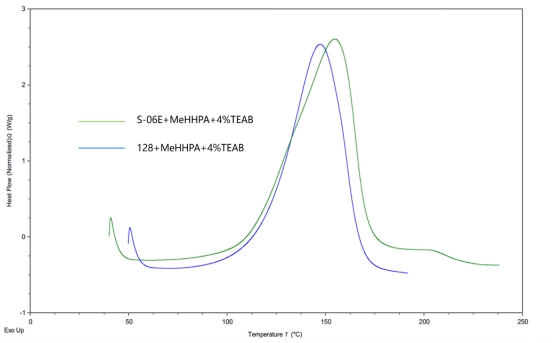
Figure 2
During the curing of epoxy resins with anhydrides, the following reactions occur under the influence of tertiary amines or quaternary ammonium salts as promoters:
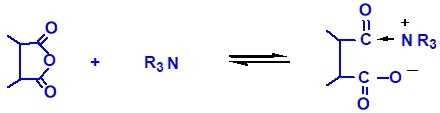
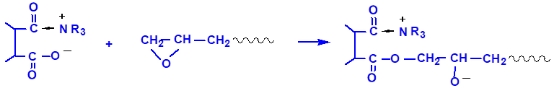
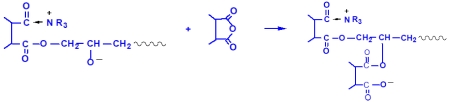
Figure 2 compares the curing kinetics curves of cycloaliphatic epoxy resins S-06E, bisphenol-A type 128 epoxy resin, and methylhexahydrophthalic anhydride. From the figure, it can be seen that when choosing the appropriate promoter and a high dosage (4% TEAB), the initial reaction temperature and peak reaction temperature of S-06E with the anhydride curing agent are close to those of the 128 resin.
Compared to amine curing agents, anhydride curing agents are less toxic and have lower volatility, but they still have some drawbacks:
Anhydrides are very easy to absorb moisture from the air, producing free acids that affect the curing rate and the performance of the cured product;
The reactivity of anhydrides with epoxy is relatively low, making it difficult to cure at room temperature;
The amounts of epoxy and anhydride need to be precisely stoichiometrically measured. If the epoxy group content is too high, it will reduce the Tg of the cured product, while too high a content of anhydride will cause issues such as a deeper color and poorer transparency of the cured product.
Compared to free radical photoinitiated curing, cationic photoinitiated curing has the following advantages:
No surface oxygen inhibition;
Greater curing depth, superior to free radical systems;
Low shrinkage, good adhesion to substrates, excellent chemical resistance, and boil resistance;
No solvent and no harmful substance emissions during curing, making it environmentally friendly.
Based on this, alicyclic epoxy photoinitiated curing systems can also develop solutions for use in composite materials, such as wind turbine blade repair. For those interested in sourcing specialty epoxy resins, there are high-quality products available at Tetra.